Cutaneous Reactions Following COVID-19 Vaccination- Cases from National Skin Centre, Singapore and A Review of Current Literature
Since COVID-19 vaccination drives commenced, there have been increasing reports of cutaneous reactions following COVID-19 vaccination.
Dr Chong Yi Rui Tricia,
Dr Heng Yee Kiat,
A/Prof Lim Yen Loo
Introduction
Since COVID-19 vaccination drives commenced, there have been increasing reports of cutaneous reactions following COVID-19 vaccination. Dermatologists may be called upon to manage these reactions and advise if further doses of vaccine can be administered.
It is not possible to definitively attribute COVID-19 vaccination as the cause of these cutaneous reactions but a suggestive temporal relationship, similar cutaneous eruption with each dose of vaccine, congruent histology, absence of other plausible causes and similar eruptions noted in other vaccinated individuals would increase the probability of COVID-19 vaccination as the cause. The pathomechanisms involved in these reactions are unclear. Skin prick tests and intradermal tests have limited utility in classifying these reactions as hypersensitive or reactogenic (1, 2).
Singapore’s main vaccination programme offered only mRNA-based COVID-19 vaccines (BNT162b2 or mRNA-1273) up until 19 October 2021. In this article, we present some of the cutaneous reactions following mRNA- based COVID- 19 vaccines seen at our centre and review relevant literature.
Delayed large local reactions
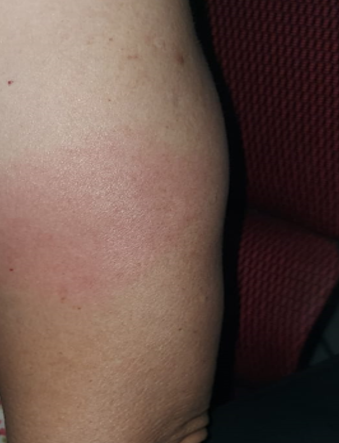
Figure 1 delayed large local reaction: A 60-year-old woman developed an itchy erythematous plaque near her injection site 4 days after each dose of mRNA-1273 vaccination. The plaques resolved spontaneously after 6 days.
Delayed large local reactions (figure 1), which usually occur 4 or more days after vaccination, was the most common cutaneous reaction reported in a study of 414 cases (3). As the self-limiting nature of delayed large local reactions was well described prior to commencement of Singapore’s vaccination drive, majority of these were seen by primary care. For the remaining cases, we provided management advice via physician-to-physician teleconsultation on secured messaging platforms (Tiger Connect®) or email.
Urticaria /angioedema/ anaphylaxis
Urticaria/ angioedema after COVID-19 vaccination is also common. Urticaria/ angioedema may be new onset or seen in individuals with chronic spontaneous urticaria or angioedema (CSU/A). These reactions usually occur within a week after vaccination and can be controlled with antihistamines (4).
Similar to Bermingham et al (5), many of us have been advising our patients with CSU/A or history of COVID-19 induced urticaria/ angioedema without systemic symptoms, to take regular antihistamines for 2 to 3 days prior to and after receiving the vaccine.
The occurrence of urticaria/ angioedema often causes individuals and their physicians to worry about vaccine allergy and hesitate to proceed with subsequent vaccination, potentially slowing the global COVID-19 vaccination effort. Fortunately, Robinson et al’s study reassured us that amongst healthcare workers who had urticaria/angioedema after their first dose of mRNA-based vaccine, many had no recurrence with their second dose (6).
Cases of anaphylaxis have been reported to occur within 15 minutes after COVID- 19 vaccination (7). According to the Centers for Disease Control and Prevention (CDC) guidelines, airway angioedema or anaphylaxis to a previous dose or component of COVID-19 vaccination is a contraindication to COVID-19 vaccination. Individuals with urticaria/ angioedema occurring within 4 hours of a previous dose of COVID- 19 vaccine should be vaccinated under the supervision of healthcare providers experienced in managing anaphylaxis/ airway angioedema (8).
Maculopapular exanthems
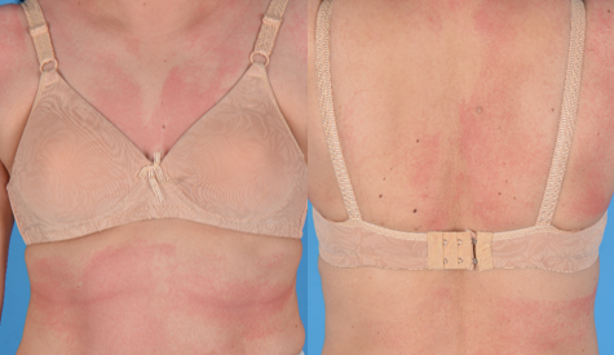
Figure 2 maculopapular exanthem: A 58-year-old woman developed a maculopapular exanthem 8 hours following her first dose of BNT162b2 COVID- 19 vaccine. No features of severe cutaneous adverse reaction were present. She was prescribed prednisolone and her exanthem resolved completely after 8 days.
Maculopapular exanthems (figure 2) are the third most common cutaneous eruption following COVID-19 vaccination. It often appears within 2 to 3 days after vaccination and resolves within 1 week (3, 9). Locally, patients who develop maculopapular exanthems without features of severe cutaneous adverse reaction are advised to proceed with further doses of the same vaccine.
Pityriasis rosea- like eruptions
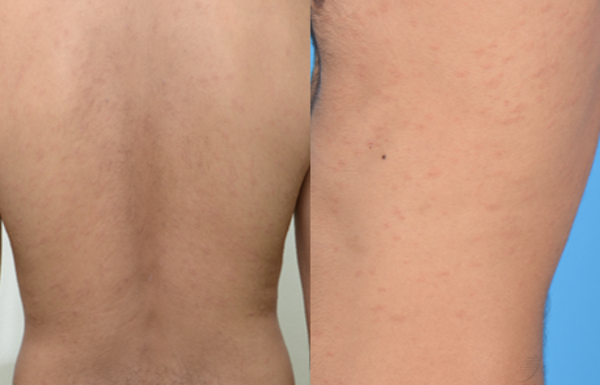
Figure 3 pityriasis rosea- like eruption: A 36- year old man developed scaly patches in a “fir-tree” distribution 21 days after his second dose of BNT162b2 COVID- 19 vaccine. He was prescribed oral erythromycin and resolution was noted 8 days after onset.
Pityriasis rosea (PR) is classically associated with Human Herpesvirus (HHV)-6, HHV-7 (10), Epstein-Barr virus (EBV) infection or vaccine-induced (11). More recently, it has been reported in COVID-19 infection and after COVID-19 vaccination (10, 12, 13). As it is regarded as a reactogenic phenomenon to the vaccine, patients with PR or a PR-like eruption (figure 3) can proceed with their next vaccine dose if due. A study reviewing the clinicopathological features of cutaneous reactions following COVID-19 vaccination suggested that PR-like eruptions may be part of the spectrum of vaccine-related eruption of papules and plaques (V-REPP). The morphological spectrum of V-REPP included papules with overlying crust, pityriasis rosea-like eruptions, and papules with subtle scale. V-REPP was noted to have varying degrees of spongiotic / interface dermatitis on histology (14).
Flare of chronic inflammatory dermatoses
Although the literature is scarce on reports of eczema flares or new onset eczema post-COVID-19 vaccination, we have seen a number of atopic dermatitis (AD) patients who report flares within a few days to 2 weeks after vaccination. Majority of these patients recovered with topical steroid and emollient application. A few patients required a rescue course of oral prednisolone, resumption or escalation of their non-steroidal immunosupressive agents. It is unclear why COVID-19 vaccination causes AD flares as the immune response post-vaccination is T helper 1 dominant.
Psoriasis exacerbation after COVID‐19 vaccination has been reported (15). We have also noted similar cases, including a pustular flare of psoriasis after vaccination. In general, we advise patients to take further doses of vaccine only when the severity of AD/psoriasis has returned to baseline with treatment.
Zoster
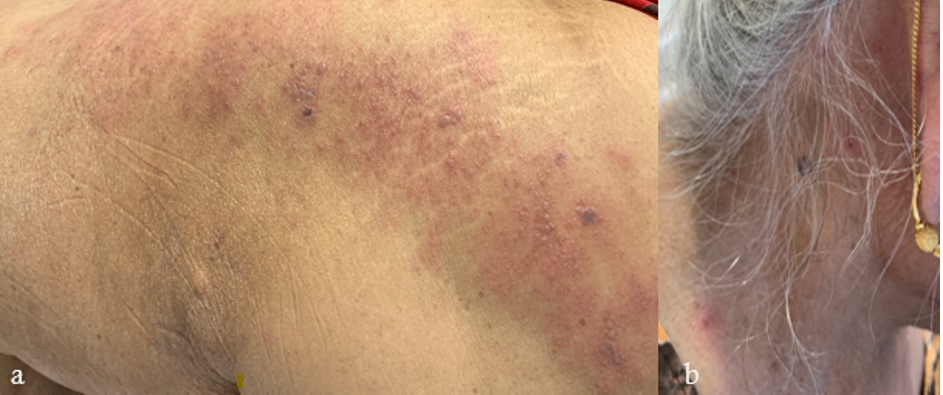
Figure 4 zoster: An 80-year-old woman with a history of breast cancer (in remission) and bullous pemphigoid (quiescent without systemic immunosuppressants), developed a few blisters on her left lower back and thigh 19 days after her first dose of BNT162b2 COVID -19 vaccination. Despite this, she proceeded to receive her second dose. Four days later, she noted multiple vesicles and bullae along the left L5 dermatome (figure 4a), bilateral posterior auricular regions (figure 4b), right side of her neck, forehead and abdomen. Varicella zoster virus polymerase chain reaction (PCR) from blister fluid was positive. Herpes simplex virus PCR was negative. She was diagnosed with disseminated zoster and admitted for intravenous acyclovir. She continues to experience post-herpetic neuralgia with residual pain along the L5 dermatome.
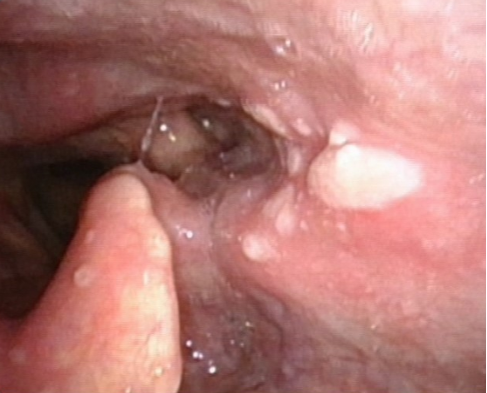
Figure 5 zoster: A 78-year-old Chinese man presented with odynophagia and an aching sensation in the left mastoid process and temporal scalp, one week after his second dose of BNT162b2 COVID-19 vaccination. Erosions in the left pharynx and laryngeal surface of the epiglottis were noted on endoscopy. Analgesia and valacyclovir 1 gram thrice a day for a week was prescribed for zoster. Two days later, he developed vesicles on the left concha and mastoid process. One week later, the laryngopharyngeal and cutaneous lesions healed. He continues to have burning pain on the left mastoid process, left temporal scalp, dizziness and new-onset left-sided hearing loss. Etoricoxib, pregabalin and cinnarizine provide some relief.
Similar to reports from other centres (9, 16), we have also observed zoster following COVID-19 vaccination (figure 4 and 5). Prompt diagnosis, treatment and deferral of vaccination until resolution of zoster may reduce morbidity. Why and how COVID-19 vaccination triggers zoster remains unclear.
Cutaneous vasculitis
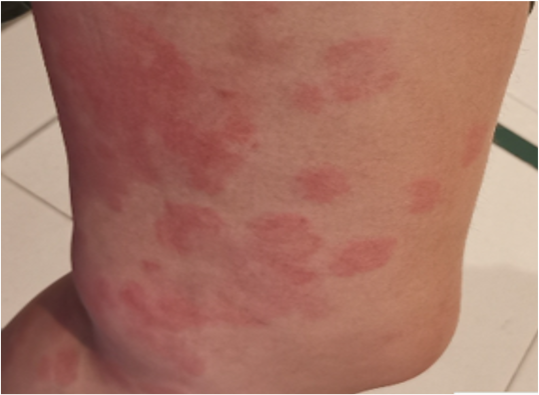
Figure 6 urticarial vasculitis: A 36- year-old woman with a background history of chronic urticaria, developed urticarial plaques that resolved with hyperpigmentation 1 day following her first dose of BNT162b2COVID-19 vaccination. Histology showed vasculitis with eosinophils. There was no systemic involvement. Disease remission was achieved with bilastine, prednisolone and cyclosporine. The patient declined further doses of COVID-19 vaccination.
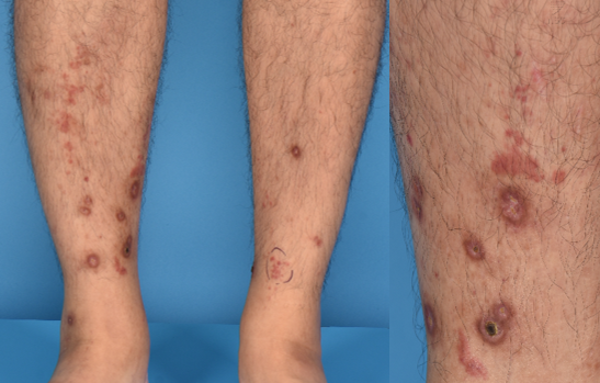
Figure 7 leucocytoclastic vasculitis: A 47- year -old man presented with palpable purpura and ulcers over his shins, 7 days after his first dose of BNT162b2COVID-19 vaccination. Histology showed leucocytoclastic vasculitis with eosinophils. He simultaneously developed new onset, persistent microscopic haematuria and is currently on follow-up with renal medicine. Treatment with colchicine and prednisolone resulted in remission of cutaneous disease. In view of renal involvement, he was advised to avoid further doses of mRNA- based COVID-19 vaccination.
Cutaneous vasculitis rarely complicates COVID-19 vaccination (3, 9). We managed one case each of urticarial vasculitis (figure 6) and leucocytoclastic vasculitis (figure 7) post COVID-19 vaccination. In general, further doses of the same vaccine in patients who have systemic involvement is discouraged. The pathomechanism of COVID-19 vaccine related vasculitis is unknown but postulations include immune complex mediated hypersensitivity reaction to the vaccine and maladaptive immune activation resulting from molecular mimicry between viral spike protein and host endothelial cells (17).
Autoimmune blistering diseases (AIBD)
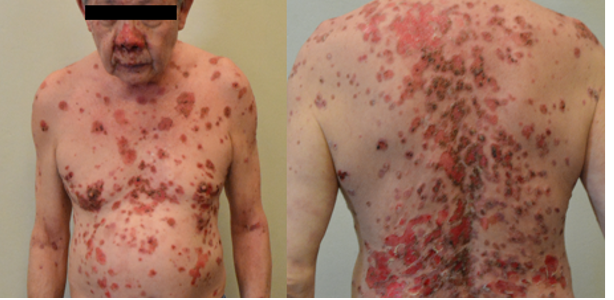
Figure 8 flare of pemphigus vulgaris: A 68- year- old man with pemphigus vulgaris in clinical remission on minimal therapy (prednisolone 2mg daily) developed buccal ulcers after his first dose of BNT162b2 COVID-19 vaccine. He proceeded with his second dose of vaccine and developed extensive erosions. He was admitted to our inpatient ward and disease control was only achieved with high- dose prednisolone and intravenous immunoglobulin.
mRNA- based COVID-19 vaccines can trigger de novo or flares of AIBD (18-20). Flares of AIBD, namely pemphigus vulgaris (figure 8) and bullous pemphigoid were seen in our clinics. Most of the cases required escalation of their immunosuppressive therapy to control the disease but we do know that such treatment may theoretically attenuate vaccine efficacy, especially when given within 2 weeks post-vaccination. Counselling patients and helping them decide if they should proceed to complete their vaccination series if flares occurred after the first dose can be challenging. Discussion points may include i) higher mortality reported in AIBD patients infected with COVID-19 (21) ii) the possibility of another AIBD flare with re-exposure to the same COVID-19 vaccine (20) iii) availability/ validity of COVID-19 antibody tests to guide to the need for further vaccine doses.
Conclusion
As illustrated, dermatologists play an important role in guiding patients with cutaneous eruptions following COVID-19 vaccination to complete their vaccination series when possible. In Singapore, patients whose risk of morbidity or mortality is significant with further doses of mRNA-based COVID-19 vaccines are allocated non-mRNA-based COVID-19 vaccines under a Special Access Route (SAR) vaccination scheme.
With booster doses planned, our collective efforts are necessary to enhance knowledge, risk stratification and management of cutaneous reactions following COVID-19 vaccination. Dermatologists may submit their cases to the American Academy of Dermatology (AAD) COVID-19 Dermatology Registry (https://www.aad.org/member/practice/coronavirus/registry), a collaborative effort between the International League of Dermatologic Societies and AAD.
References
- Stone CA, Jr., Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533-40.e8.
- Bianchi L, Biondi F, Hansel K, Murgia N, Tramontana M, Stingeni L. Skin tests in urticaria/angioedema and flushing to Pfizer-BioNTech SARS-CoV-2 vaccine: Limits of intradermal testing. Allergy. 2021;76(8):2605-7.
- McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46-55.
- Grieco T, Maddalena P, Sernicola A, Muharremi R, Basili S, Alvaro D, et al. Cutaneous adverse reactions after COVID-19 vaccines in a cohort of 2740 Italian subjects: An observational study. Dermatol Ther. 2021:e15153.
- Bermingham WH, Ardern-Jones MR, Huissoon AP, Krishna MT. Forewarned is forearmed: chronic spontaneous urticaria as a potential risk to effective SARS-CoV-2 vaccine uptake and global public health. Br J Dermatol. 2021;185(4):838-9.
- Robinson LB, Fu X, Hashimoto D, Wickner P, Shenoy ES, Landman AB, et al. Incidence of Cutaneous Reactions After Messenger RNA COVID-19 Vaccines. JAMA Dermatol. 2021;157(8):1000-2.
- Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine – United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):46-51.
- CDC. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States [Available from:https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#Appendix-B.
- Català A, Muñoz-Santos C, Galván-Casas C, Roncero Riesco M, Revilla Nebreda D, Solá-Truyols A, et al. Cutaneous reactions after SARS-COV-2 vaccination: A cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2021.
- Merhy R, Sarkis AS, Stephan F. Pityriasis rosea as a leading manifestation of COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35(4):e246-e7.
- Drago F, Ciccarese G, Javor S, Parodi A. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30(3):544-5.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(9):e436-e7.
- Veraldi S, Spigariolo CB. Pityriasis rosea and COVID-19. J Med Virol. 2021;93(7):4068.
- McMahon DE, Kovarik CL, Damsky W, Rosenbach M, Lipoff JB, Tyagi A, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J Am Acad Dermatol. 2021.
- Sotiriou E, Tsentemeidou A, Bakirtzi K, Lallas A, Ioannides D, Vakirlis E. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021.
- Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021.
- Cavalli G, Colafrancesco S, De Luca G, Rizzo N, Priori R, Conti F, et al. Cutaneous vasculitis following COVID-19 vaccination. Lancet Rheumatol. 2021.
- Tomayko MM, Damsky W, Fathy R, McMahon DE, Turner N, Valentin MN, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148(3):750-1.
- Young J, Mercieca L, Ceci M, Pisani D, Betts A, Boffa MJ. A case of bullous pemphigoid after the SARS-CoV-2 mRNA vaccine. J Eur Acad Dermatol Venereol. 2021.
- Damiani G, Pacifico A, Pelloni F, Iorizzo M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated? J Eur Acad Dermatol Venereol. 2021;35(10):e645-e7.
- Kridin K, Schonmann Y, Weinstein O, Schmidt E, Ludwig RJ, Cohen AD. The risk of COVID-19 in patients with bullous pemphigoid and pemphigus: A population-based cohort study. J Am Acad Dermatol. 2021;85(1):79-87.














