COVID-19 and the Skin
Reviews of selected publications
Collection of investigations, articles, researches about skin diseases associated with COVID-19
COVID-19 & DERMATOLOGY
The Impact of the Coronavirus Disease (COVID-19) on the Health and Social Needs of Sex Workers in Singapore
Rayner Kay Jin Tan, Vanessa Ho, Sherry Sherqueshaa, Wany Dee, Jane Mingjie Lim, Jamie Jay-May Lo, Alvin Kuo Jing Teo, Caitlin Alsandria O’Hara, Clarence Ong, Ann Hui Ching, Mee Lian Wong
Archives of Sexual Behavior https://doi.org/10.1007/s10508-021-01951-8
Reviewed by Drs Wu Xiaotian & Martin Chio, National Skin Centre Singapore
This article by R Tan et al studied the impact of the COVID-19 pandemic on sex workers in Singapore. It was conducted using a mixed methods model – comprising of a qualitative phase involving interviews with various stakeholders in the sex work industry to identify various important themes, which were subsequently investigated through a quantitative phase by surveying sex workers.
In the qualitative phase, the researchers found that the closure of brothels and other venues where sex work usually occurs as well as the implementation of the circuit breaker (lockdown) during the COVID-19 pandemic has adversely impacted sex workers. Sex workers may also miss out on COVID-19 relief measures and opportunities due to the stigma of being a sex worker and the lack of proper paperwork to qualify for relief. To adapt to the new challenging environment, some sex workers are trying to find part time/ad-hoc work, while others are out of work or carrying on sex work illegally.
In the quantitative phase, the researchers found that the pandemic has resulted in a significant loss of income for the sex workers, resulting in increase in food insecurity and housing insecurity. Sex workers also reported a decrease in access to medical or healthcare services as well as an increase in sexual compromise (though not specifically elaborated upon). Moreover, transgender sex workers appear to be disproportionately affected with a greater loss of income, and in turn, increased food and housing insecurity.
The study does have several limitations. Firstly, the number of survey participants eventually recruited was lower than the initial target to achieve a 95% confidence interval and 5% error margin. Moreover, the population may not be entirely representative as a disproportionate number of local (i.e. Singapore citizens/PRs) sex workers were recruited. The study is also subject to recall bias.
However, despite the limitations, this study is a good starting point for stakeholder agencies to figure out how to offer more assistance to a particularly vulnerable population during this Covid-19 pandemic.
Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases.
Devon E. McMahon, Erin Amerson, Misha Rosenbach, Jules B. Lipoff, Danna Moustafa, Anisha Tyagi, Seemal R. Desai, Lars E. French, Henry W. Lim, Bruce H. Thiers, George J. Hzura, Kimberly G. Blumenthal, Lindy P. Fox, Esther E. Freeman
Journal of the American Academy of Dermatology 85.1 (2021): 46-55.
Reviewed by Dr Cheng Hui Mer, National Skin Centre Singapore
Covid-19 vaccination at a national level is one of the key strategies against its spread. As populations globally began to receive inoculations at a staggering pace, an urgent need to acquire knowledge about possible reactions from these vaccines arose. The United States was amongst the first to approve the use of mRNA vaccines in December 2020, and a registry previously used for the collection of cutaneous manifestations of SARS-CoV-2 was expanded to collect Covid-19 vaccination cutaneous reactions. With this as the background, this study aimed to collect cases of cutaneous side effects of mRNA Covid-19 vaccines, namely the Pfizer (BNT162b2) and Moderna (mRNA-1273), to describe the morphology and timing of cutaneous reactions, and to understand differences in cutaneous reactions between the two vaccine doses to guide vaccine counselling.
Using an online registry with case entry opened to healthcare workers only, 414 unique cases were collected of one or more cutaneous reactions to Moderna (83%) or Pfizer (17%) Covid-19 vaccines from December 24 2020 to February 14 2021. Of note, at the time of analysis, vaccination was administered to healthcare workers and elderly participants only. Amongst the participants included, median age was 44 years old, 90% were female, 78% were white and the cases were primarily from the US (98%).
343 and 71 unique reports of cutaneous manifestations after Moderna and Pfizer vaccinations, respectively, were classified into 5 categories: local site reaction, urticaria, morbilliform, delayed large local reaction, and erythromelalgia. (Figure below)
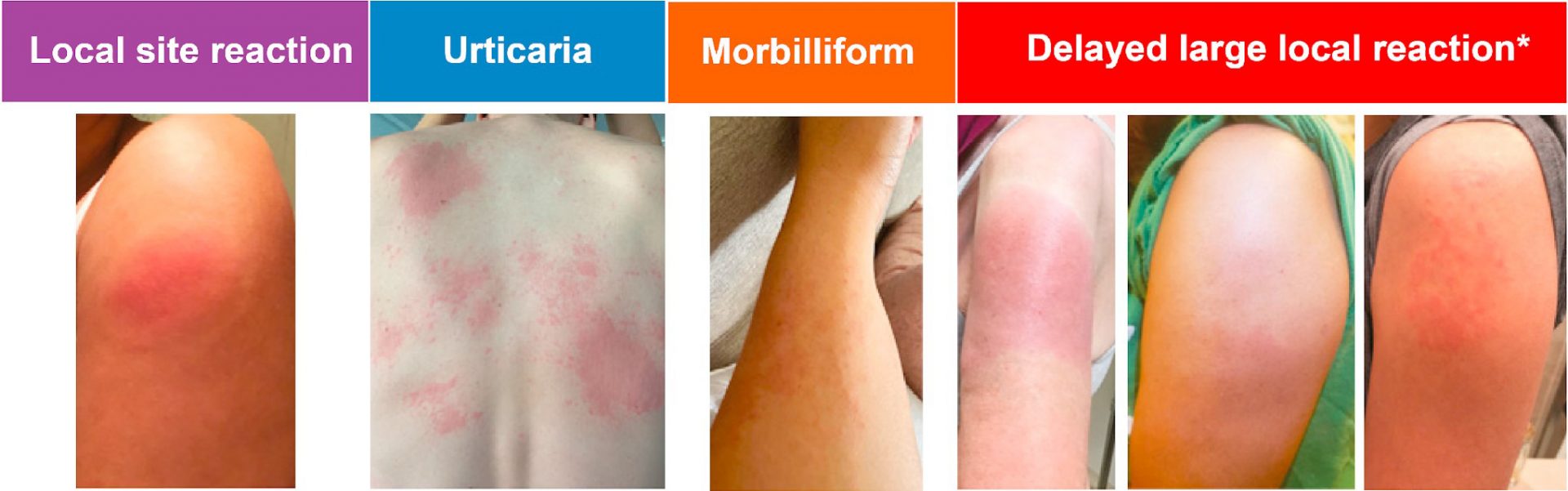
Timing information was available for 350 (85%) of the 414 records, which showed that delayed large local arm reactions occurred primarily after
Moderna vaccination (94%) at a median of 7 days (IQR 7-8) after the first vaccine, lasting a median of 4 days (IQR 3-6). Reaction occurred more quickly after 2nd vaccine dose at median of 2 days (IQR 1-3) and lasted median of 3 days (IQR 2-5). Importantly, no urticaria or angioedema reports after the first dose were immediate in onset and all came after 1 day or more.
As set out by the authors, this study provided important initial insight into the range of Covid-19 vaccine-related cutaneous manifestations and presented useful data for counselling of patients who have had a cutaneous reaction to the first dose of vaccine. Some limitations of this study were its inability to rule out concomitant Covid-19 infections, or drug reactions, in particular to analgesics which may be taken as pre-medication for Covid-19 vaccinations. Most vaccine reactions reported in this study were of Moderna vaccines but due to the design of the study, it could not be concluded if Moderna vaccines were more likely to result in cutaneous reactions than Pfizer vaccines. For the same reason, incidence of cutaneous reactions post-vaccination could not be calculated. Last but not least, the data collected appeared to capture a subset of the general population (white, female, middle-aged), possibly reflecting disparities to vaccine and healthcare access and at the same time, reducing the study’s external validity.
Overall, this study was exceptional in spearheading efforts in the understanding of cutaneous reactions from Covid-19 vaccinations but given its myriad presentations, there remains much to be known. Further work is necessary in understanding reactions that occur in skin of colour and in the management of more severe cutaneous reactions, particularly in patients with pre-existing dermatologic conditions.
Consensus on a set of criteria to attribute causality of cutaneous manifestations to Covid-19 vaccination is also an area of need as this may have medicolegal implications, particularly when government and/or insurance financial reimbursements come into play. The transparency of this criteria impacts doctor-patient trust and public confidence in vaccine safety.














